PROJECT FOCUS : GRAIL
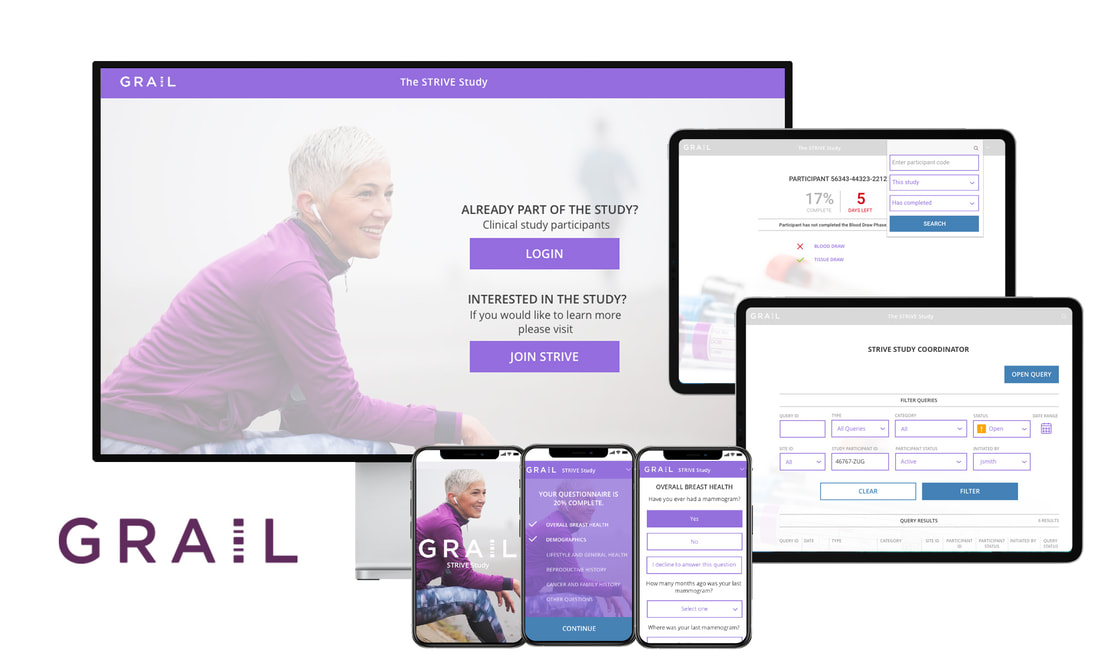
About the Client: Grail is a health technology company focused on early cancer detection. I worked specifically on the STRIVE Study, which enrolled approximately 100,000 women at the time of their screening mammogram and is used to validate a blood test for the early detection of multiple cancer types. Participants completed a health questionnaire and provided a blood sample around the time of their screening mammogram and will be followed for up to five years to capture clinical outcome data, including cancer diagnoses.
My Roles: project lead, design lead, UX design, liaison with development
My Roles: project lead, design lead, UX design, liaison with development
Core Project Components:
Participant View: The questionnaire that participants are invited to take part in at the time of their screening mammogram.
Admin View: Administrator's view of the participants questionnaire results, and next qualifying steps. A full database of participant's information so the administrator may continue to gather qualifying data and keep participants moving along in the process.
Lab Tech View: Detailing the blood draw including collecting samples, entering participant notes at time of draw, scanning and labeling samples, and shipping samples.
Study Coordinator View: Full database of all validated participants and their status. The main function besides viewing includes query management, including the functionality to flag and inquire potential discrepancies within a participant’s questionnaire, and/or flag missing elements relating to their participation. It also includes internal tracking of query status and follow-up, as well as filtering capabilities and status views so the coordinator can quickly navigate the participants' records and find information they need.
Project Objectives:
Participant View: The questionnaire that participants are invited to take part in at the time of their screening mammogram.
Admin View: Administrator's view of the participants questionnaire results, and next qualifying steps. A full database of participant's information so the administrator may continue to gather qualifying data and keep participants moving along in the process.
Lab Tech View: Detailing the blood draw including collecting samples, entering participant notes at time of draw, scanning and labeling samples, and shipping samples.
Study Coordinator View: Full database of all validated participants and their status. The main function besides viewing includes query management, including the functionality to flag and inquire potential discrepancies within a participant’s questionnaire, and/or flag missing elements relating to their participation. It also includes internal tracking of query status and follow-up, as well as filtering capabilities and status views so the coordinator can quickly navigate the participants' records and find information they need.
Project Objectives:
- Design a questionnaire that is user friendly and provides incentive for participant follow-through.
- The need to keep participants' information confidential while keeping specific information tied to each participant.
- Applying a consistent look/feel and flow to a varied range of use cases.
- Designing around and adapting evolving brand guidelines and standards.
- Fast-track iterations and a streamlined workflow to meet required delivery milestones.
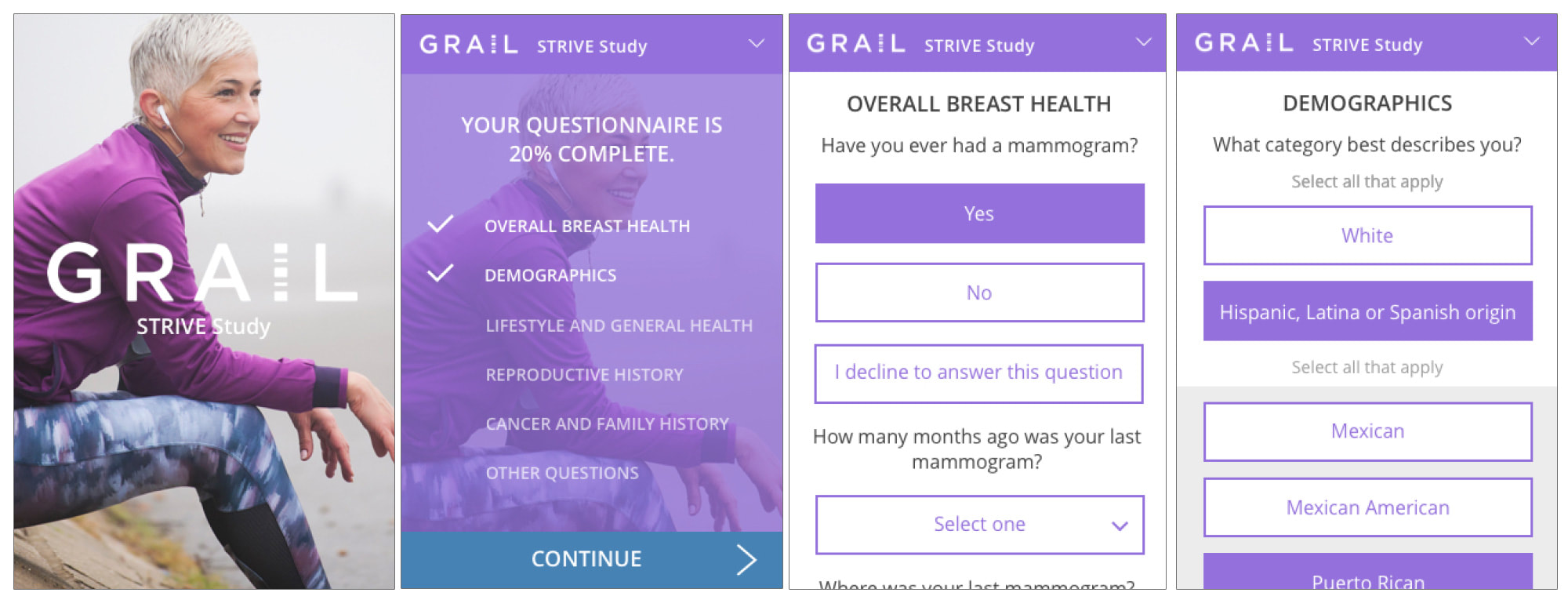
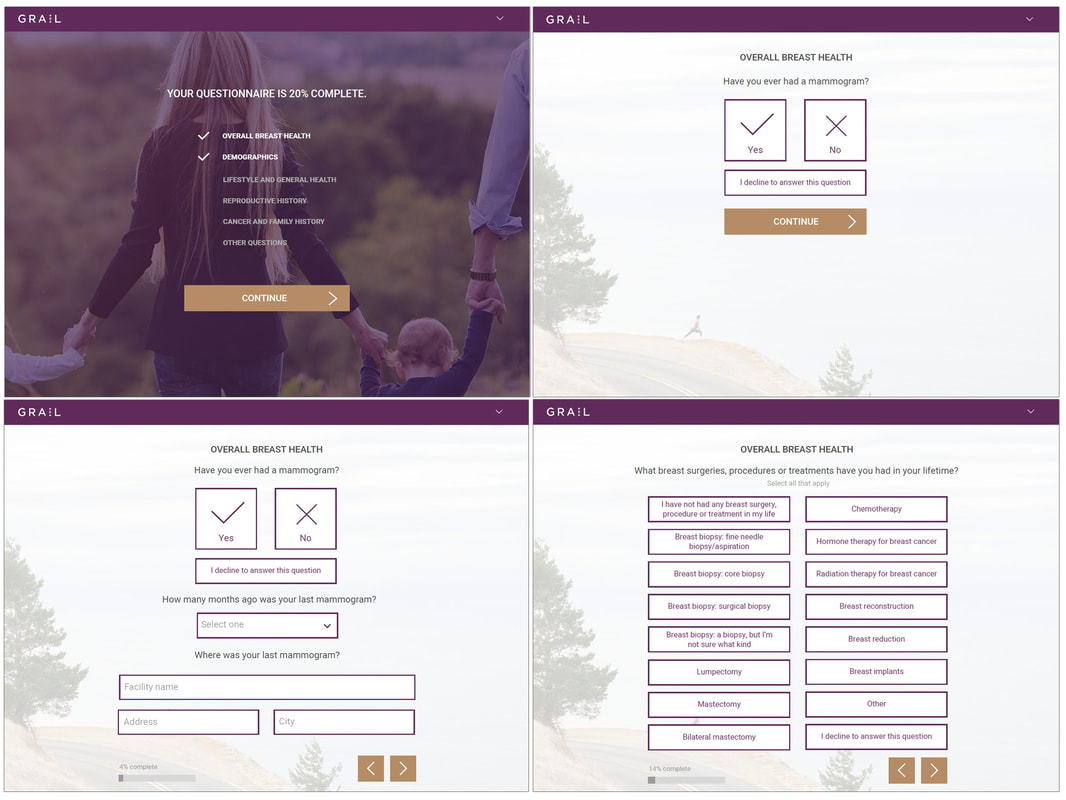
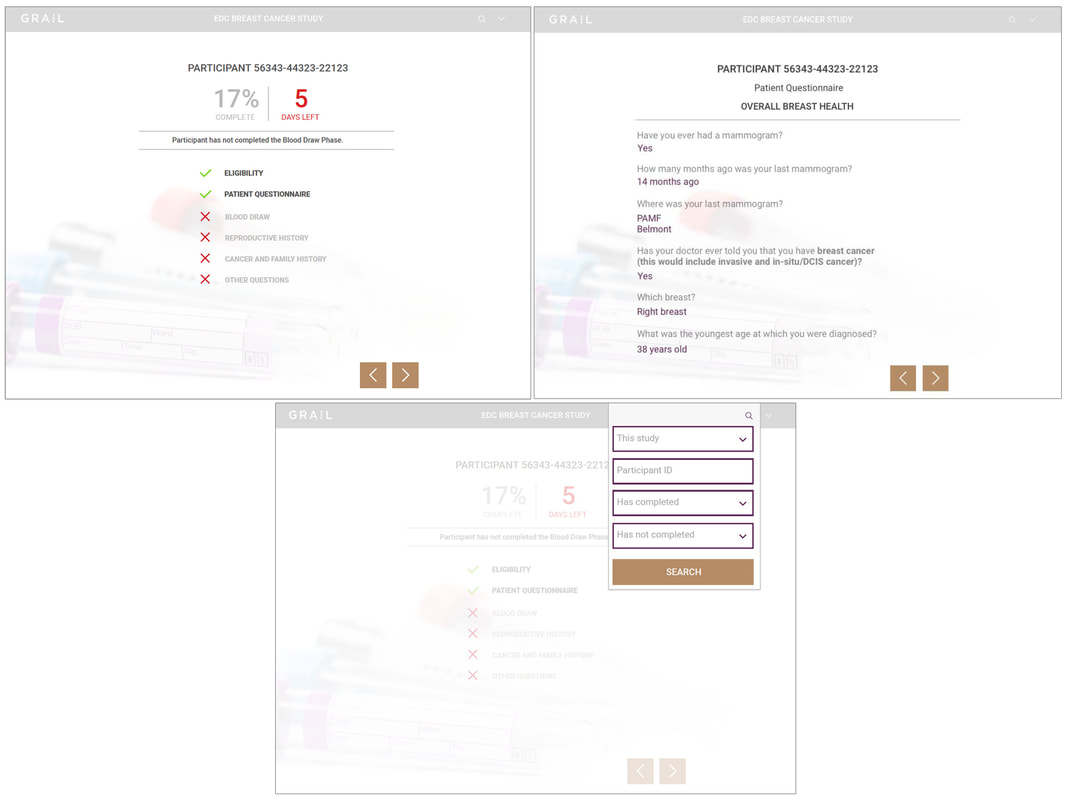
Participant view
The Participant View was the first phase and involved the most straight-forward component: the elective participant questionnaire flow provided at time of mammogram. My goal was to provide a straightforward, dynamic design that was very easy to follow and as streamlined as possible to encourage participation and completion.
Examples of data types were provided to me, and I worked with stakeholders to present solutions that allowed participants to compete the questionnaires as efficiently and accurately as possible as I mocked up examples of the flow. The branding and style guidelines shifted in the first 2 phases which dictated a pivot in visual design.
Examples of data types were provided to me, and I worked with stakeholders to present solutions that allowed participants to compete the questionnaires as efficiently and accurately as possible as I mocked up examples of the flow. The branding and style guidelines shifted in the first 2 phases which dictated a pivot in visual design.
| Grail Questionnaire Mobile Layout | |
| File Size: | 714 kb |
| File Type: | |
Admin view
The Admin View was a summary of participant questionnaire results and supplemental materials. This comprised a participant database of information so the administrator may keep the participants moving along in the process within the given timeframe.
| Grail Admin View | |
| File Size: | 1252 kb |
| File Type: | |
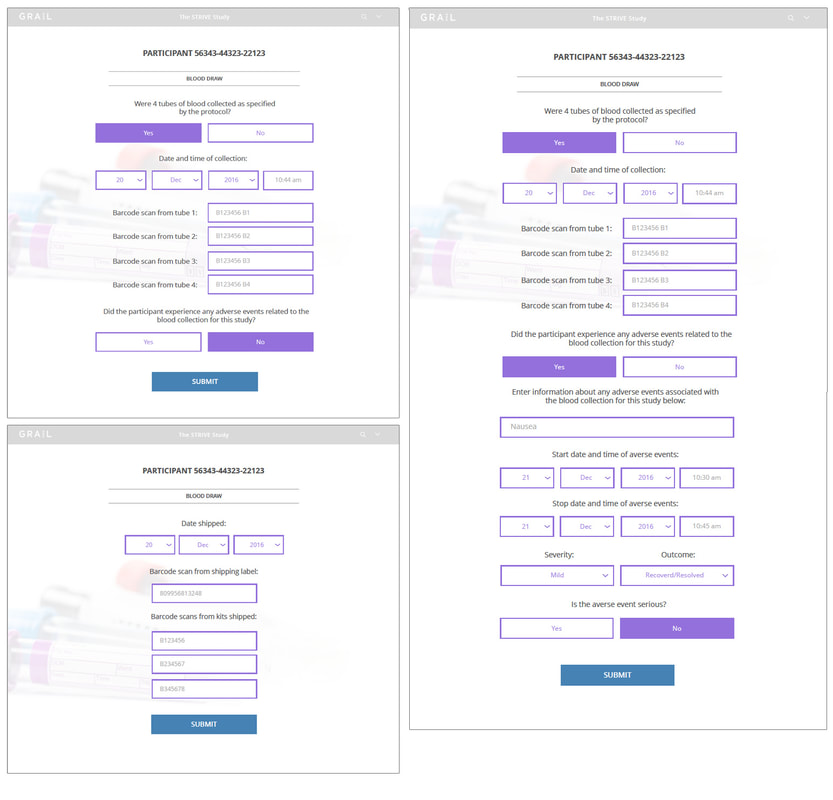
LAB TECH VIEW
The Lab Tech View detailed the blood draw process including the tasks of collecting samples, entering participant notes at time of the blood draw, scanning and labeling samples, and shipping samples. The main objective was to accurately and confidentially record and ship blood samples.
| Grail Lab Tech View | |
| File Size: | 1181 kb |
| File Type: | |
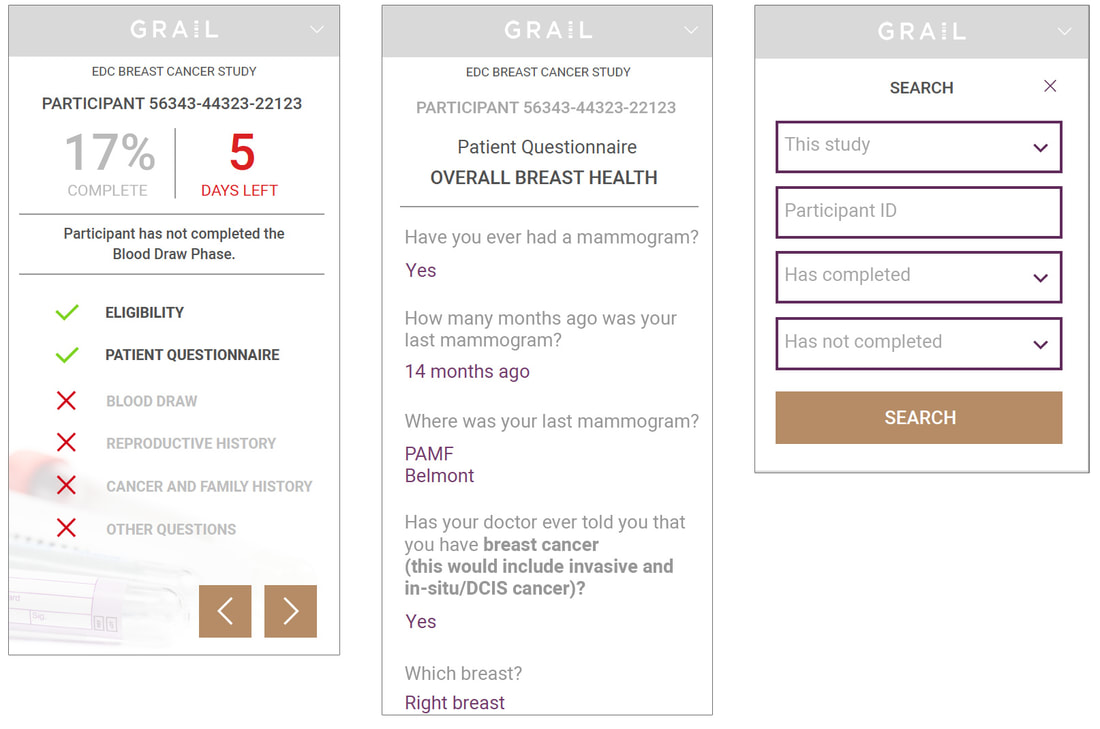
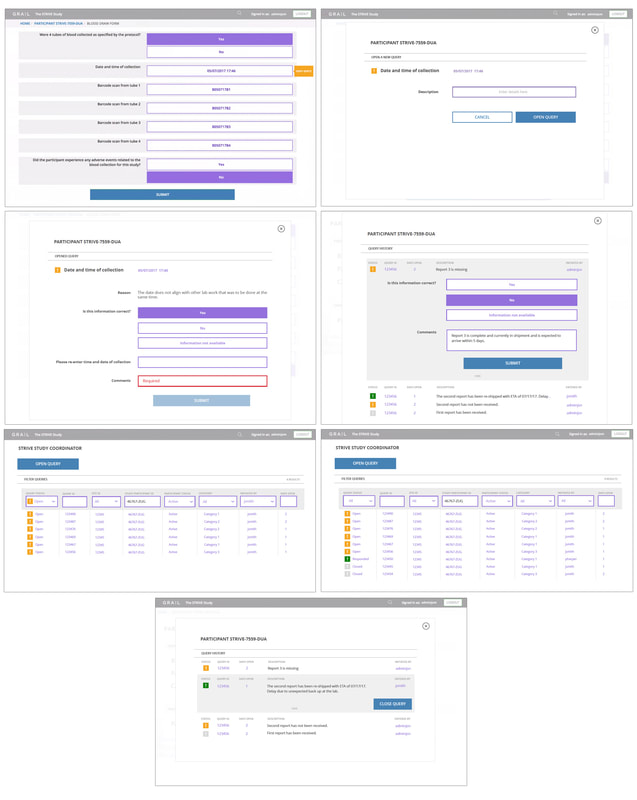
Study Coordinator VIEW
The Study Coordinator View was the most robust of all the phases. It is comprised of the full database of all participants and status. Query management was a major interactive element of this view, including the functionality to flag and inquire potential discrepancies within a participant’s questionnaire, and/or flag missing elements needed for the participant's involvement in the study . It also included internal tracking of query status and follow-up. The database required clear filtering capabilities and status views so the coordinator may follow up on open queries. These requirements translated into key objectives:
- Clean views at the top level to quickly indicate open items.
- Ability to dig deep, view and communicate within the system.
- Manage participant’s activity efficiently while capturing meticulous records.
- Effectively qualify participants for the study.
| Study Coordinator View | |
| File Size: | 1294 kb |
| File Type: | |